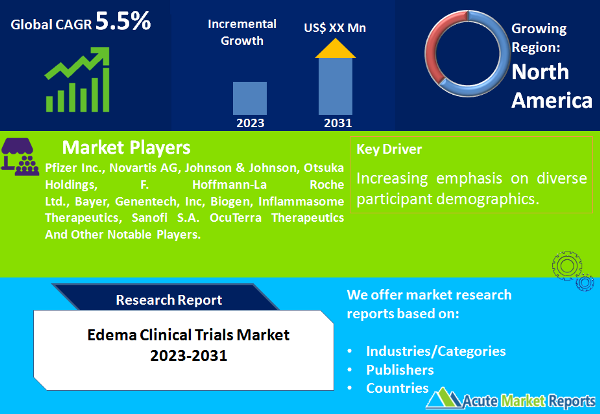
The edema clinical trials market is undergoing significant advancements and is expected to grow at a CAGR of 5.5% during the forecast period of 2024 to 2032, fueled by evolving research methodologies and a growing focus on diverse participant demographics. As we transition from 2023, marked by substantial revenues, to the forecast period of 2024 to 2032, the Edema Clinical Trials market is poised for continued expansion. The ongoing innovative strategies employed in clinical trials are expected to shape the landscape of edema treatment, addressing the complexities of diverse patient populations and paving the way for more effective and patient-centric interventions.
Advancements in Therapeutic Approaches
The foremost driver in the Edema Clinical Trials market is the continuous evolution of therapeutic approaches. As evidenced by the emergence of novel drug candidates and interventions, ongoing clinical trials are exploring innovative mechanisms to address edema more effectively. Investigational treatments targeting specific pathways, such as vascular permeability and lymphatic function, have demonstrated promising results in early-phase trials. These advancements reflect a concerted effort to enhance treatment efficacy and reduce adverse effects, paving the way for more targeted and personalized interventions.
Increased Emphasis on Pediatric Research
A crucial driver shaping the landscape of edema clinical trials is the increased emphasis on pediatric research. Recognizing the unique challenges and distinct physiological responses in pediatric populations, recent trials have focused on developing evidence-based interventions specifically tailored to children. This shift is evident in the inclusion of pediatric cohorts in multi-phase trials, ensuring the safety and efficacy of edema treatments for this demographic. By addressing the unmet medical needs of pediatric patients, these trials contribute to a more comprehensive understanding of edema management across diverse age groups.
Growing Integration of Digital Technologies
Another key driver propelling the Edema Clinical Trials market is the growing integration of digital technologies. From electronic data capture systems to wearable devices monitoring edema-related parameters, clinical trials are leveraging digital innovations to enhance data accuracy, streamline patient monitoring, and improve overall trial efficiency. Real-time data collection and analysis enable researchers to make informed decisions, accelerating the pace of clinical trials and contributing to the timely development of effective edema treatments.
Challenges in Patient Recruitment and Retention
A significant restraint in the Edema Clinical Trials market is the persistent challenge of patient recruitment and retention. Despite the advancements in therapeutic approaches and digital technologies, enrolling a diverse and representative participant pool remains a complex task. Evidence supporting this restraint includes instances of prolonged trial timelines and increased costs due to difficulties in recruiting a sufficient number of eligible participants. Additionally, the high attrition rates observed in some trials underscore the need for innovative strategies to enhance patient engagement and retention throughout the trial duration.
By Phase: Phase III trials Dominates the Market
In 2023, the Edema Clinical Trials market experienced substantial revenue across different phases, with Phase III trials generating the highest revenue. However, Phase IV trials demonstrated the highest CAGR during the forecast period from 2024 to 2032, indicating a shift towards post-marketing research and continued exploration of long-term treatment outcomes. This dynamic segmentation underscores the evolving nature of clinical research in edema, emphasizing both advanced stages and post-marketing surveillance.
By Participant: The Adults Segment Dominates the Market
Market segmentation by participants in 2023 showcased robust revenue streams from adults, reflecting the predominant focus on this demographic in clinical trials. However, Pediatrics demonstrated the highest CAGR during the forecast period, highlighting a growing recognition of the importance of pediatric research in addressing edema in younger populations. Simultaneously, Geriatrics maintained substantial revenue, illustrating a comprehensive approach that considers the unique characteristics of elderly patients in clinical trial design.
North America Remains the Global Leader
Geographically, the Edema Clinical Trials market exhibited diverse trends in 2023, with North America leading in revenue generation. However, the Asia-Pacific region showcased the highest CAGR, reflecting a surge in clinical trial activities and increasing investment in research infrastructure. Balancing revenue and CAGR, these geographic trends underscore the global nature of edema clinical trials, with diverse regions contributing uniquely to research advancements.
Market Competition to Intensify during the Forecast Period
In the competitive landscape, top players such as Pfizer Inc., Novartis AG, Johnson & Johnson, Otsuka Holdings, F. Hoffmann-La Roche Ltd., Bayer, Genentech, Inc, Biogen, Inflammasome Therapeutics, Sanofi S.A. and OcuTerra Therapeutics are driving innovation and shaping the future of edema clinical trials. These key players reported substantial revenues in 2023, expecting continued growth from 2024 to 2032. Their strategic focus on developing advanced therapeutic approaches, emphasizing pediatric research, and leveraging digital technologies positions them as influential entities in the edema clinical trials landscape. As the market evolves, these players are anticipated to play a pivotal role in advancing clinical research, ultimately contributing to the development of more effective treatments for edema.
Historical & Forecast Period
This study report represents analysis of each segment from 2022 to 2032 considering 2023 as the base year. Compounded Annual Growth Rate (CAGR) for each of the respective segments estimated for the forecast period of 2024 to 2032.
The current report comprises of quantitative market estimations for each micro market for every geographical region and qualitative market analysis such as micro and macro environment analysis, market trends, competitive intelligence, segment analysis, porters five force model, top winning strategies, top investment markets, emerging trends and technological analysis, case studies, strategic conclusions and recommendations and other key market insights.
Research Methodology
The complete research study was conducted in three phases, namely: secondary research, primary research, and expert panel review. key data point that enables the estimation of Edema Clinical Trials market are as follows:
Market forecast was performed through proprietary software that analyzes various qualitative and quantitative factors. Growth rate and CAGR were estimated through intensive secondary and primary research. Data triangulation across various data points provides accuracy across various analyzed market segments in the report. Application of both top down and bottom-up approach for validation of market estimation assures logical, methodical and mathematical consistency of the quantitative data.
| ATTRIBUTE | DETAILS |
|---|---|
| Research Period | 2022-2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Year | 2022 |
| Unit | USD Million |
| Segmentation | |
Phase
| |
Participant
| |
Study Design
| |
Type
| |
|
Region Segment (2022-2032; US$ Million)
|
Key questions answered in this report