The central nervous system (CNS) biomarkers market is a rapidly growing industry that involves the use of biomarkers to diagnose and monitor various neurological disorders. CNS biomarkers can be measured in cerebrospinal fluid (CSF), blood, or urine, and can help in the early detection and diagnosis of disorders such as Alzheimer's disease, Parkinson's disease, Multiple Sclerosis, and others.The central nervous system biomarkers market is expected to grow at a steady CAGR of around 10.2% from 2026 to 2034.
Increasing Prevalence of Neurological Disorders
The increasing prevalence of neurological disorders is a major driver for the CNS biomarkers market. According to the World Health Organization (WHO), around one billion people worldwide are affected by neurological disorders, with approximately six million deaths each year. In the US alone, the prevalence of Alzheimer's disease is projected to increase from 5.8 million in 2021 to 13.8 million by 2050. There is growing evidence that early detection and intervention can improve outcomes for patients with neurological disorders. CNS biomarkers can provide valuable diagnostic and prognostic information, leading to earlier and more accurate diagnosis and personalized treatment plans. This has led to increased demand for CNS biomarkers and is driving the growth of the market.
Technological Advancements in Biomarker Detection
Advancements in technology for biomarker detection are also driving the growth of the CNS biomarkers market. The development of new techniques for biomarker detection and analysis, such as mass spectrometry, microarray analysis, and imaging technologies, has led to improved sensitivity and specificity in biomarker detection. For example, the development of amyloid imaging has enabled the detection of beta-amyloid protein, a biomarker for Alzheimer's disease, in living patients. This has led to earlier and more accurate diagnosis of the disease, and has spurred the development of new treatments.
Increasing Number of Clinical Trials for CNS Disorders
The increasing number of clinical trials for CNS disorders is also driving the growth of the CNS biomarkers market. Clinical trials are essential for the development of new treatments and therapies for neurological disorders, and biomarkers can play a critical role in identifying and monitoring patient populations for these trials. For example, in a recent clinical trial for Alzheimer's disease, biomarkers were used to select patients for the trial and to monitor treatment efficacy. This approach led to a more targeted and efficient trial, with improved outcomes for patients. As the number of clinical trials for CNS disorders continues to increase, the demand for CNS biomarkers is also expected to grow.
Lack of Standardization and Regulatory Challenges in Biomarker Development and Validation
Biomarkers are complex and dynamic biological indicators that require rigorous validation and standardization to ensure accuracy and reproducibility. However, there is currently a lack of consensus on standardization and validation methods for CNS biomarkers. This makes it difficult to compare and interpret results across different studies and limits the utility of CNS biomarkers in clinical practice. Furthermore, the regulatory landscape for biomarkers is complex and varies across different jurisdictions. In the US, for example, the FDA has established guidelines for the development and validation of biomarkers for drug development, but these guidelines are not mandatory and do not cover all types of biomarkers. This can lead to uncertainty and delays in the approval process for biomarker-based therapies. The lack of standardization and regulatory challenges in biomarker development and validation are significant barriers to the growth of the CNS biomarkers market. Addressing these challenges will require increased collaboration among stakeholders, including researchers, regulators, and industry leaders, to establish consensus on standards and guidelines for biomarker development and validation.
Safety Biomarkers: Ensuring Drug Safety and Patient Well-being
The central nervous system (CNS) biomarkers market is witnessing significant growth due to advancements in medical research, the rising prevalence of neurological disorders, and the increasing demand for personalized medicine. The market can be categorized into different types of biomarkers, including safety biomarkers, efficacy biomarkers, validation biomarkers, and others. Safety biomarkers play a crucial role in the central nervous system (CNS) biomarkers market by assessing the safety profile and potential adverse effects of drugs or therapeutic interventions on the CNS. These biomarkers provide valuable insights into the impact of treatments on the nervous system and help ensure patient safety during clinical trials and drug development processes. With increasing concerns about patient safety and the need for reliable and effective treatments, there is a growing emphasis on drug safety assessment. Safety biomarkers enable researchers and clinicians to monitor the potential neurotoxicity and adverse effects of drugs on the CNS, allowing for early identification and mitigation of risks. Technological advancements, such as genomics, proteomics, and imaging techniques, have accelerated the discovery and development of safety biomarkers. These advancements have enabled researchers to identify specific biomarkers that indicate neurotoxicity or adverse effects, leading to improved safety assessments and better decision-making during drug development. Regulatory agencies, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), have established guidelines for safety assessment in clinical trials. These guidelines emphasize the importance of safety biomarkers in evaluating the potential risks associated with new drugs, further driving the demand for safety biomarkers in the CNS biomarkers market.
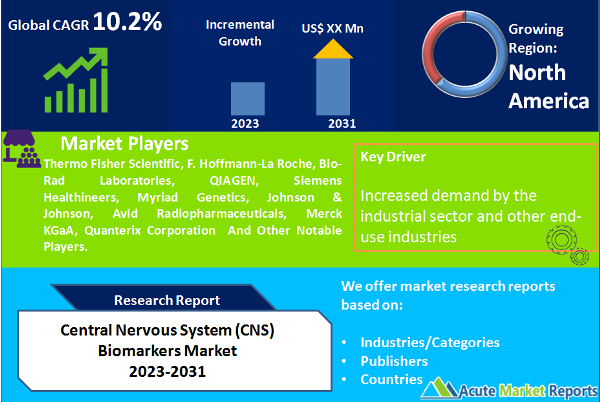
North America: Dominating the Central Nervous System Biomarkers Market
North America holds the highest revenue in the central nervous system (CNS) biomarkers market due to its well-established healthcare infrastructure, high prevalence of neurological disorders, advanced research capabilities, favorable regulatory environment, and substantial healthcare spending. The region's robust healthcare system, including advanced facilities and research institutions, facilitates efficient biomarker research and development. Additionally, the relatively high prevalence of neurological disorders in North America drives the demand for CNS biomarkers. The region's leading research institutions and biotechnology companies contribute to innovation and the development of novel biomarkers. Furthermore, the supportive regulatory environment and significant investments in healthcare infrastructure and research further propel the revenue generation in the North American CNS biomarkers market.
Competitive Analysis
Competitive trends in the central nervous system (CNS) biomarkers market are characterized by increasing focus on precision medicine, strategic collaborations and partnerships, technological advancements, emphasis on neurodegenerative disorders, and market expansion strategies. Companies are investing in the development of biomarkers for personalized treatment approaches and are forming collaborations to leverage expertise and expand their market reach. Technological advancements in genomics, proteomics, metabolomics, and imaging techniques are driving innovation in biomarker detection and analysis. Neurodegenerative disorders, such as Alzheimer's and Parkinson's diseases, are receiving significant attention in biomarker research. Market players are also expanding their presence in emerging markets and forming strategic alliances to tap into new customer bases and capitalize on the growing demand for CNS biomarkers. These competitive trends shape the dynamic landscape of the CNS biomarkers market and drive advancements in diagnosis, treatment, and patient care. Central nervous system (CNS) biomarkers market are driven by top market players who contribute to advancements and innovations. Companies such as Thermo Fisher Scientific, F. Hoffmann-La Roche, Bio-Rad Laboratories, QIAGEN, Siemens Healthineers, Myriad Genetics, Johnson & Johnson, Avid Radiopharmaceuticals, Merck KGaA, and Quanterix Corporation are actively involved in research and development, strategic collaborations, and technological advancements. These market players aim to enhance their biomarker offerings, expand their product portfolios, and gain a competitive edge. Their contributions shape the competitive landscape of the CNS biomarkers market, driving innovation, improving diagnostic capabilities, and advancing patient care in the field of neurological disorders.
Historical & Forecast Period
This study report represents analysis of each segment from 2024 to 2034 considering 2025 as the base year. Compounded Annual Growth Rate (CAGR) for each of the respective segments estimated for the forecast period of 2026 to 2034.
The current report comprises of quantitative market estimations for each micro market for every geographical region and qualitative market analysis such as micro and macro environment analysis, market trends, competitive intelligence, segment analysis, porters five force model, top winning strategies, top investment markets, emerging trends and technological analysis, case studies, strategic conclusions and recommendations and other key market insights.
Research Methodology
The complete research study was conducted in three phases, namely: secondary research, primary research, and expert panel review. key data point that enables the estimation of Central Nervous System (CNS) Biomarkers market are as follows:
Market forecast was performed through proprietary software that analyzes various qualitative and quantitative factors. Growth rate and CAGR were estimated through intensive secondary and primary research. Data triangulation across various data points provides accuracy across various analyzed market segments in the report. Application of both top down and bottom-up approach for validation of market estimation assures logical, methodical and mathematical consistency of the quantitative data.
| ATTRIBUTE | DETAILS |
|---|---|
| Research Period | 2024-2034 |
| Base Year | 2025 |
| Forecast Period | 2026-2034 |
| Historical Year | 2024 |
| Unit | USD Million |
| Segmentation | |
Type
|
|
Application
|
|
End User
|
|
|
Region Segment (2024-2034; US$ Million)
|
Key questions answered in this report