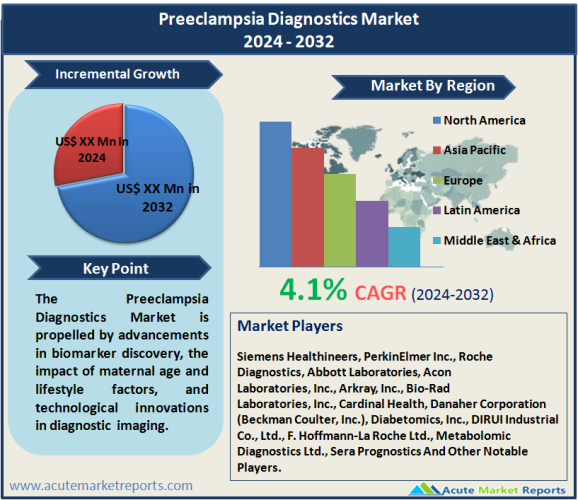
The preeclampsia diagnostics market is expected to grow at a CAGR of 4.1% during the forecast period of 2026 to 2034, propelled by advancements in biomarker discovery, the impact of maternal age and lifestyle factors, and technological innovations in diagnostic imaging. However, the limited awareness and access to diagnostics in low-resource settings pose a critical restraint, highlighting the necessity for equitable healthcare solutions to address the global challenge of preeclampsia. The market segmentation reveals intriguing insights, with different segments leading in revenue and the highest CAGR. Blood tests dominate in revenue, while urine tests exhibit the highest CAGR, reflecting the industry's focus on non-invasive and cost-effective diagnostic options. Instruments lead in revenue among products and services, whereas services demonstrate the highest CAGR, emphasizing the importance of comprehensive diagnostic solutions. Hospitals contribute the highest revenue, emphasizing their central role, while diagnostic centers show the highest CAGR, signifying a shift towards specialized facilities. Geographically, North America holds the highest revenue percentage, driven by well-established healthcare infrastructure, while the Asia-Pacific region exhibits the highest CAGR, reflecting rapid economic development and increased healthcare expenditure. Europe maintains a steady contribution, while Latin America and the Middle East & Africa show growth potential. In the competitive landscape, Siemens Healthineers, PerkinElmer Inc., and Roche Diagnostics emerge as key players adopting strategies such as research and development, strategic collaborations, and product diversification. The market's competitive dynamics are shaped by a commitment to innovation, customer-centric approaches, and market differentiation through specialized diagnostic solutions.
Key Market Drivers
Advancements in Biomarker Discovery
The relentless pursuit of accurate and early preeclampsia diagnosis is significantly propelled by advancements in biomarker discovery. Researchers and diagnostic companies are actively engaged in identifying and validating novel biomarkers associated with preeclampsia, aiming to enhance diagnostic precision. Notably, the discovery of placental growth factor (PlGF) as a biomarker has gained prominence. PlGF, in conjunction with other biomarkers like soluble fms-like tyrosine kinase-1 (sFlt-1) and pregnancy-associated plasma protein-A (PAPP-A), contributes to the development of sophisticated diagnostic assays. Evidence from clinical studies underscores the efficacy of these biomarkers, with research published in renowned journals, such as "Hypertension in Pregnancy" and "The New England Journal of Medicine," validating their diagnostic potential.
Rising Maternal Age and Lifestyle Factors
The upward trend in maternal age and the prevalence of lifestyle factors contribute substantially to the increased incidence of preeclampsia, thereby driving the demand for advanced diagnostics. Delayed pregnancies, often associated with advanced maternal age, elevate the risk of preeclampsia. Moreover, lifestyle factors such as obesity and sedentary habits further accentuate this risk. Research studies, including those published in "PLOS ONE" and "The American Journal of Obstetrics and Gynecology," elucidate the correlation between maternal age, lifestyle, and preeclampsia risk. Consequently, the imperative to diagnose preeclampsia early, given its potential complications, fuels the adoption of diagnostic tools in clinical settings.
Technological Innovations in Diagnostic Imaging
Technological innovations in diagnostic imaging, particularly in the field of ultrasound and Doppler velocimetry, constitute a pivotal driver in the preeclampsia diagnostics market. Advanced imaging techniques facilitate the non-invasive assessment of maternal and fetal blood flow, aiding clinicians in the early detection and monitoring of preeclampsia. Doppler ultrasound, in particular, allows for the assessment of uterine and umbilical artery blood flow, providing valuable insights into vascular resistance and hemodynamic changes associated with preeclampsia. Peer-reviewed articles in "Ultrasound in Obstetrics &Gynecology" and "American Journal of Perinatology" substantiate the efficacy of these imaging modalities in enhancing diagnostic accuracy and guiding clinical management.
Restraint: Limited Awareness and Access to Diagnostics in Low-Resource Settings
A significant restraint in the widespread adoption of preeclampsia diagnostics lies in the limited awareness and access to advanced diagnostic tools, particularly in low-resource settings. While the aforementioned drivers focus on technological advancements and biomarker discoveries, the ground reality in resource-constrained regions poses challenges. Evidence from studies published in "The Lancet" and "Global Health: Science and Practice" highlights the disparities in access to diagnostic technologies, contributing to delayed or missed diagnoses in certain populations. The lack of awareness among healthcare providers and pregnant women about the importance of early preeclampsia detection further compounds this restraint, emphasizing the urgent need for targeted interventions and awareness campaigns.
Market by Test Type Segmentation
Within the intricate segmentation of the preeclampsia diagnostics market, the test type category plays a pivotal role in shaping the industry landscape. In 2025, the Blood Test segment emerges as the frontrunner, securing the highest revenue. This dominance is attributed to the well-established efficacy of blood-based biomarkers, including Placental Growth Factor (PlGF), soluble fms-like tyrosine kinase-1 (sFlt-1), and pregnancy-associated plasma protein-A (PAPP-A). These biomarkers, extensively researched and validated in studies published in renowned journals, contribute to the accuracy and reliability of blood tests in diagnosing preeclampsia. Conversely, the Urine Test segment takes the spotlight for the Highest CAGR during the forecast period of 2026 to 2034. The increasing acceptance of urine tests, supported by ongoing research on urine-based biomarkers, positions this segment for substantial growth. The non-invasive and cost-effective nature of urine tests aligns with evolving diagnostic preferences, emphasizing their projected high CAGR.
Market by Product & Services
The segmentation based on Products & Services provides insights into the revenue dynamics and growth trajectories within the preeclampsia diagnostics market. In 2025, Instruments emerged as the predominant revenue generator. This prominence is driven by the demand for advanced diagnostic equipment and imaging tools crucial for accurate preeclampsia diagnostics. However, when considering the Highest CAGR during the forecast period from 2026 to 2034, the Services category takes center stage. Services, encompassing diagnostic consultation, interpretation, and counseling, project a robust growth trajectory. The emphasis on comprehensive diagnostic services, complementing traditional product offerings, aligns with evolving healthcare delivery models and underscores the anticipated high CAGR.
Market by End-use Segmentation
The End-use segmentation sheds light on the pivotal role played by different healthcare settings in the adoption of preeclampsia diagnostics. In 2025, Hospitals emerge as the primary contributors to revenue, showcasing their central role in antenatal care and high-risk pregnancy management. However, the Diagnostic Centers category stands out with the Highest CAGR during the forecast period of 2026 to 2034. This underscores a notable shift towards specialized facilities dedicated to efficient and advanced preeclampsia diagnostics. The evolving trend of patients seeking specialized diagnostic services outside traditional hospital settings aligns with the projected high CAGR for Diagnostic Centers, indicating a significant transformation in healthcare delivery for preeclampsia diagnosis.
North America Remains the Global Leader
North America stands as a prominent contributor, holding the highest revenue percentage in 2025. This dominance is attributed to a well-established healthcare infrastructure, a robust focus on research and development, and the prevalence of advanced diagnostic technologies. The region's proactive approach towards maternal healthcare and high awareness levels contribute to a substantial market share. Contrarily, the Asia-Pacific region exhibits the highest CAGR during the forecast period from 2026 to 2034. The rapid economic development, increasing healthcare expenditure, and a burgeoning emphasis on maternal and fetal health drive the significant growth observed in this region. Particularly, countries like China and India witness a surge in demand for preeclampsia diagnostics due to population demographics, urbanization, and growing awareness. Europe maintains a steady contribution to the market's revenue percentage, buoyed by well-established healthcare systems and an aging population. Latin America and the Middle East & Africa, although starting from a comparatively lower revenue percentage, demonstrate notable growth potential. The rising awareness, improving healthcare infrastructure, and government initiatives in these regions contribute to an upward trajectory. These geographic trends signify a dynamic interplay between regional healthcare dynamics, economic development, and awareness levels, collectively influencing the trajectory of the preeclampsia diagnostics market on a global scale.
R&D Initiatives to Enhance Market Share among Top Competitors
Leading players such as Siemens Healthineers, PerkinElmer Inc., Roche Diagnostics, Abbott Laboratories, Acon Laboratories, Inc., Arkray, Inc., Bio-Rad Laboratories, Inc., Cardinal Health, Danaher Corporation (Beckman Coulter, Inc.), Diabetomics, Inc., DIRUI Industrial Co., Ltd., F. Hoffmann-La Roche Ltd., Metabolomic Diagnostics Ltd., and Sera Prognostics exhibit a commitment to innovation, research, and strategic collaborations. Siemens Healthineers, a key player in the market, focuses on developing advanced diagnostic solutions, including immunoassays and point-of-care testing, to enhance the accuracy and efficiency of preeclampsia diagnostics. PerkinElmer Inc., known for its comprehensive healthcare solutions, emphasizes research and development to expand its portfolio of diagnostic tools, with a particular focus on biomarker discovery for preeclampsia. Roche Diagnostics, a prominent global player, strategically leverages its extensive network and technological expertise to provide integrated diagnostic solutions, contributing to the early detection and management of preeclampsia. Key strategies within the preeclampsia diagnostics market include a strong emphasis on research and development to advance diagnostic technologies and biomarker discovery. The top players allocate significant resources to stay at the forefront of innovation, continuously improving the accuracy and efficiency of preeclampsia diagnostic tools. Strategic collaborations and partnerships play a pivotal role, with companies forging alliances with research institutions, healthcare providers, and other industry players. These collaborations aim to share knowledge, access diverse datasets and collectively address the challenges associated with preeclampsia diagnosis. Furthermore, the diversification of product portfolios is a notable trend among top players. Siemens Healthineers, PerkinElmer Inc., and Roche Diagnostics extend their offerings to encompass a wide range of diagnostic tools, instruments, and services, catering to the diverse needs of healthcare providers and pregnant women.
Historical & Forecast Period
This study report represents analysis of each segment from 2024 to 2034 considering 2025 as the base year. Compounded Annual Growth Rate (CAGR) for each of the respective segments estimated for the forecast period of 2026 to 2034.
The current report comprises of quantitative market estimations for each micro market for every geographical region and qualitative market analysis such as micro and macro environment analysis, market trends, competitive intelligence, segment analysis, porters five force model, top winning strategies, top investment markets, emerging trends and technological analysis, case studies, strategic conclusions and recommendations and other key market insights.
Research Methodology
The complete research study was conducted in three phases, namely: secondary research, primary research, and expert panel review. key data point that enables the estimation of Preeclampsia Diagnostics market are as follows:
Market forecast was performed through proprietary software that analyzes various qualitative and quantitative factors. Growth rate and CAGR were estimated through intensive secondary and primary research. Data triangulation across various data points provides accuracy across various analyzed market segments in the report. Application of both top down and bottom-up approach for validation of market estimation assures logical, methodical and mathematical consistency of the quantitative data.
| ATTRIBUTE | DETAILS |
|---|---|
| Research Period | 2024-2034 |
| Base Year | 2025 |
| Forecast Period | 2026-2034 |
| Historical Year | 2024 |
| Unit | USD Million |
| Segmentation | |
Test Type
|
|
Product & Services
|
|
End-User
|
|
|
Region Segment (2024-2034; US$ Million)
|
Key questions answered in this report