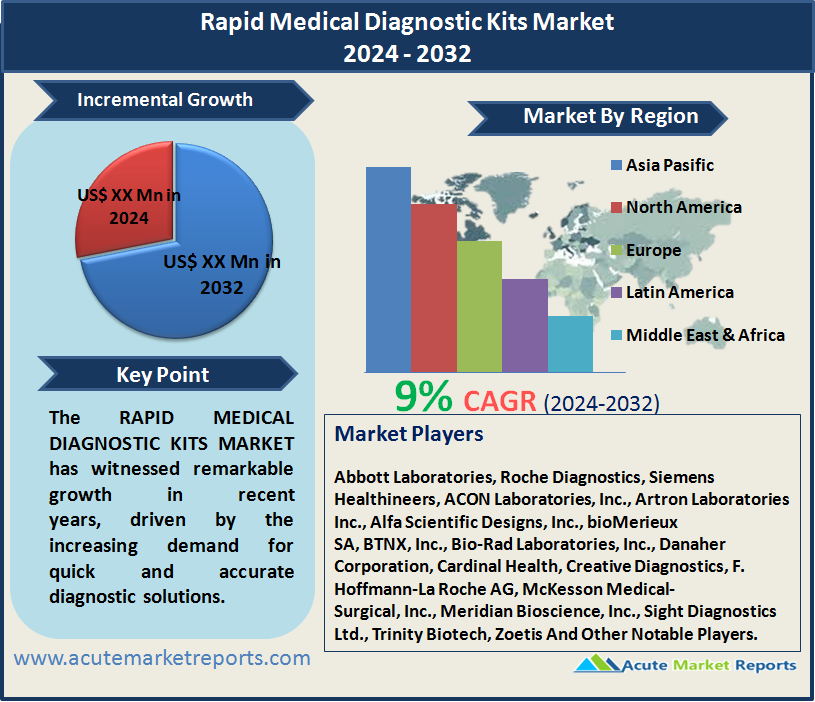
The rapid medical diagnostic kits market has witnessed remarkable growth in recent years, driven by the increasing demand for quick and accurate diagnostic solutions. The rapid medical diagnostic kits market is expected to grow at a CAGR of 9% during the forecast period of 2026 to 2034, fueled by the increasing emphasis on Point-of-Care Testing (POCT), global health threats, and advancements in diagnostic technologies. While challenges in test specificity and accuracy pose restraints, the market's segmentation provides valuable insights into the preferences and priorities of healthcare professionals and consumers. Geographic trends underscore the varying degrees of adoption across regions, and competitive trends highlight the key players and strategies shaping the market. As the market progresses into the forecast period from 2026 to 2034, the continued focus on rapid and accurate diagnostics, especially in the wake of global health challenges, is expected to drive the expansion of the rapid medical diagnostic kits market.
Growing Emphasis on Point-of-Care Testing (POCT): Revolutionizing Healthcare Delivery through Rapid Diagnostics
The rapid medical diagnostic kits market is experiencing robust growth due to the growing emphasis on Point-of-Care Testing (POCT) across healthcare settings. Evidence includes the increasing adoption of rapid diagnostic kits in hospitals, clinics, and other healthcare facilities to facilitate timely and on-site diagnosis. This driver is underscored by the potential of rapid diagnostic kits to revolutionize healthcare delivery by providing quick results, enabling immediate decision-making, and improving patient outcomes. As evidenced by the integration of POCT into various medical specialties and the rising number of healthcare providers incorporating rapid diagnostic solutions, this driver is expected to be a significant catalyst for market growth from 2026 to 2034.
Global Health Threats and Pandemic Preparedness: Rapid Kits as Essential Tools in Containing Infectious Diseases
The Rapid Medical Diagnostic Kit market is thriving due to global health threats and the need for effective pandemic preparedness. Evidence includes the pivotal role played by rapid diagnostic kits in the detection and containment of infectious diseases, such as the COVID-19 pandemic. This driver is highlighted by the increased awareness of the importance of rapid and accurate diagnostics in responding to public health emergencies. As evidenced by the surge in demand for rapid COVID-19 testing and the development of diagnostic kits for emerging infectious diseases, this driver is expected to contribute significantly to market growth during the forecast period.
Advancements in Diagnostic Technologies: Innovative Technologies Enhancing Speed and Accuracy
The rapid medical diagnostic kits market is witnessing substantial growth driven by continuous advancements in diagnostic technologies. Evidence includes the development of innovative technologies that enhance the speed, sensitivity, and accuracy of rapid diagnostic tests. This driver is emphasized by the integration of novel detection methods and the incorporation of microfluidics and biosensor technologies into rapid diagnostic kits. As evidenced by the expanding range of diagnostic applications and the increasing focus on precision medicine, this driver is expected to play a pivotal role in shaping the rapid medical diagnostic kits market landscape from 2026 to 2034.
Challenges in Test Specificity and Accuracy: Balancing Speed with Precision in Rapid Diagnostics
A notable restraint in the rapid medical diagnostic kits market is the challenges associated with ensuring test specificity and accuracy. Evidence includes instances where rapid diagnostic tests may produce false-positive or false-negative results, leading to potential misdiagnosis and inappropriate treatment decisions. This restraint is further emphasized by the need to balance the speed of results with the precision required for accurate diagnostics. As evidenced by occasional discrepancies in test performance and the ongoing efforts to enhance the accuracy of rapid diagnostic kits, addressing these challenges requires a comprehensive approach that considers both the urgency of results and the reliability of diagnostic information.
By Product: Professional Kits Segment Dominates the Market
In 2025, the rapid medical diagnostic kits market demonstrated substantial revenue from the Professional Kits segment, reflecting the high demand for accurate and specialized diagnostic solutions in clinical settings. Simultaneously, the Over Counter (OTC) Kits segment exhibited the highest CAGR during the forecast period from 2026 to 2034, indicating a surge in consumer preference for convenient and self-administered diagnostic tests. This nuanced segmentation illustrates the diverse applications and user preferences within the market, with both professional and OTC kits contributing uniquely to the overall growth of the rapid medical diagnostic kits market.
By Technology: Lateral Flow Technology Dominates the Market
The rapid medical diagnostic kits market showcased significant revenue from the Lateral Flow technology segment in 2025, emphasizing the widespread use of this simple and cost-effective detection method. Concurrently, other technologies, including Agglutination and Solid Phase, exhibited the highest CAGR during the forecast period, signifying a shift towards more sophisticated and diverse diagnostic approaches. This comprehensive segmentation highlights the evolving landscape of diagnostic technologies, with both established and emerging methods meeting distinct market demands.
APAC Remains the Global Leader
Geographically, the rapid medical diagnostic kits market exhibited diverse trends in 2025, with the Asia-Pacific region leading in revenue generation and the highest CAGR. This reflects the region's proactive approach towards adopting rapid diagnostic solutions, driven by a growing healthcare infrastructure and increasing awareness of the benefits of early diagnosis. North America also contributed significantly to revenue, driven by a well-established healthcare system and continuous investments in innovative diagnostic technologies. Europe, while demonstrating a robust market, maintained a slightly lower CAGR compared to Asia-Pacific, indicating a steady but slightly slower adoption of rapid diagnostic kits. This geographic segmentation offers insights into regional dynamics, highlighting key trends, and providing a comprehensive outlook for the forecast period from 2026 to 2034.
Market Competition to Intensify during the Forecast Period
In the competitive landscape, top players such as Abbott Laboratories, Roche Diagnostics, Siemens Healthineers, ACON Laboratories, Inc., Artron Laboratories Inc., Alfa Scientific Designs, Inc., bioMerieux SA, BTNX, Inc., Bio-Rad Laboratories, Inc., Danaher Corporation, Cardinal Health, Creative Diagnostics, F. Hoffmann-La Roche AG, McKesson Medical-Surgical, Inc., Meridian Bioscience, Inc., Sight Diagnostics Ltd., Trinity Biotech, and Zoetis have played pivotal roles in shaping the rapid medical diagnostic kits market. These companies, with their extensive portfolios of rapid diagnostic solutions, strong global presence, and continuous investments in research and development, have been key contributors to the market's growth. In 2025, these industry leaders demonstrated robust revenues, and their strategic initiatives position them as key influencers in the market's future growth. Looking ahead to the forecast period from 2026 to 2034, these companies are expected to maintain their prominence and contribute to the ongoing evolution of the rapid medical diagnostic kits market.
Historical & Forecast Period
This study report represents analysis of each segment from 2024 to 2034 considering 2025 as the base year. Compounded Annual Growth Rate (CAGR) for each of the respective segments estimated for the forecast period of 2026 to 2034.
The current report comprises of quantitative market estimations for each micro market for every geographical region and qualitative market analysis such as micro and macro environment analysis, market trends, competitive intelligence, segment analysis, porters five force model, top winning strategies, top investment markets, emerging trends and technological analysis, case studies, strategic conclusions and recommendations and other key market insights.
Research Methodology
The complete research study was conducted in three phases, namely: secondary research, primary research, and expert panel review. key data point that enables the estimation of Rapid Medical Diagnostic Kits market are as follows:
Market forecast was performed through proprietary software that analyzes various qualitative and quantitative factors. Growth rate and CAGR were estimated through intensive secondary and primary research. Data triangulation across various data points provides accuracy across various analyzed market segments in the report. Application of both top down and bottom-up approach for validation of market estimation assures logical, methodical and mathematical consistency of the quantitative data.
| ATTRIBUTE | DETAILS |
|---|---|
| Research Period | 2024-2034 |
| Base Year | 2025 |
| Forecast Period | 2026-2034 |
| Historical Year | 2024 |
| Unit | USD Million |
| Segmentation | |
Product
|
|
Technology
|
|
Application
|
|
End-Use
|
|
|
Region Segment (2024-2034; US$ Million)
|
Key questions answered in this report